ICS, or the incident command
system is system of communication during disaster/incidents. In other words, this is the chain of
information and the order in which people are informed about events. This is something that was taken much more
serious during a series of fires in California in the 1970’s.
It was determined that the fire was so
devastating, not because of lack of manpower or resources, but from lack of
communication. That being said, of
course today we have a much easier way of communicating. Everyone has mobile phones, emails, etc. So today communication is much easier and
much more efficient. Regardless,
communication still plays just as big of a rule, or maybe even a bigger role
now, in emergencies. The amount of time
it takes for emergency responders to respond to an emergency can often be the
difference between life and death. An
example of were lack of communication cost lives was in 9/11. Some lack of communication is always expected. You cannot expect all of New York City to be
aware of a disaster as soon as it happens.
However, many people lost there life’s simply because they did not know
what was happening. There are stories of
people gathering up their belongings in the towers and never coming out because
of the time they wasted. Also people in
the surrounding areas were not necessarily instantly aware of what was taking
place. If the chain of command in the
towers was improved it could have saved life.
We see a similar scenario in other tragedies like Hurricane
Katrina. Some people were just never
told to evacuate. Because of this
reason, it is estimated that many people lost their life’s. Many people were in fact told to leave, and
refused because they did not fully understand the magnitude of the situation. ICS is used on many different scales. Whether it is in private business or the
United States government. There are many
intricacies to the ICS. Including long
and short term planning. In large-scale tragedies
it could take an unforeseen amount of time to get everything cleaned up. Hurricane Katrina took years and it is still
not completely cleaned up.
The ICS can
include grotesque procedures, including those telling how to deal with a
massive amount of fatalities and what to do with the bodies. Unfortunately this is an inevitable part of
tragedies. Procedures like this are
crucial to deal with the scenarios as effectively as possible and save as many life’s
as possible. However in a mass chaos there are many unforeseen events that can disrupt the planed ICS. No matter how much planning and preparation
goes into an ICS there will always be some form of confusion. But that’s why so much time and effort is
goes into preventing disasters. In an
occupational setting, that is why we have safety regulations. Massive disasters can occur as result of
negligence for safety. There are
countless chemicals, equipment, and compressed gases that can very easily cause
a large scale disaster. For this reason,
ICS and its contributing standards are extremely important.
Thursday, April 28, 2016
Tuesday, April 26, 2016
PCB's
PCBs, or PolychlorinatedBiphenyls, are a group of environmentally harmful compounds that used to be
produced in the United States. A PCB
molecule is any of 209 chemical compounds called congeners. For something to be classified as a PCB, one
to ten chlorine atoms must be attached to a biphenyl molecule.
PCBs were manufactured in the United States,
Europe, and Japan under the trade name “Aroclor”. Monsanto was the company that produced
“Aroclor”. PCB’s used to be in just about everything. Whether it be plastics, paints, rubber
products, coolants, electrical components or anything dealing with the
manufacturing process. They are favorable in products for their thermal and
chemical resistance. PCBs have a very
high boiling point, usually above 270 degrees Celsius. This is what makes them so useful for heat
resistant applications. For this reason
they were often used in high temperature lubricants. Many plastic parts for automobiles, and other
equipment that are close to a heat source such as an engine contained PCB’s. For the same reason, PCB’s were widely used
in electrical equipment. Transformers
and lining of wires often require being I insulated with heat resistant
material.
This requirement makes PCB’s a
perfect match. While banned in many countries, like the U.S., Canada, and a lot
of the European Union, many developing countries still use PCB’s in production
of their products. For the same reasons that make PCB’s so
useful, they are also harmful to the environment. Because they are so resistant to heat and
other factors, they also do not break down in the environment over time. This means that they accumulate at a very
rapid rate. This is particular evident
in our water ways. The animal that is
considered to have the highest PCB concentration is the orca whale. But in more general terms, aquatic life
generally has the highest level of PCB’s.
Predatory fish/mammals have the highest levels due to bioaccumulation. This is because they are eating lower life
forms every day. The PCB’s that their
prey has absorbed is than brought into the body of the predatory animal. Because PCB’s do not break down easy, they
stay in the animal’s body. This can have
many adverse health effects. Just some
of the effects include mothers having premature births, cancer, heart disease,
and pretty much anything else you can think of relating to a chronic
condition. Now this all sounds bad, no
one wants animals to be sick. But the
concerning part about this is that humans are eating these animals. So we are also suffering from bioaccumulation. PCB’s are thought to have the same effect in
humans as on marine mammals. This is
concerning because such a large portion of the fish we eat come from the ocean.
In particular salmon, which is a
predatory fish. So if bioaccumulations
happening in salmon, it is even more magnified in humans, since we are actually
eating a predator. Overall, we need to
completely stop the use PCBs worldwide.
It does not matter if the U.S. is no longer using PCB’s if other nations
still are. PCB’s drift thousands of
miles and spread all across the world in are oceans.
Sunday, April 3, 2016
Fit Testing
Fit testing is a very
important part of making sure employees respiratory devices are working
properly. The purpose of the fit test is
to ensure that the mask has an airtight seal around the face of the individual
wearing it. If the fit is not right, than
whatever material trying to be kept out may be breathed in by the person
wearing the mask. This is a reason for
concern, particularly when many dangerous gases don’t have a scent or very many
warning signs. Filtering particulates,
gases, or anything else out is a vital task of any filter respirator. An airtight seal is also vital with a
supplied air respirator. Fit tests can be done in a variety of ways. Either using an electronic machine that
detects the particles coming into the mask or simply using a very strong
scent/taste test to tell if are leaking in around the seal. Both test have some similarities but are very
different. The cheaper method of testing
is the taste/smell test. This method
does not require the high-end digital equipment that the other test
requires.
That being said it is also
less precise but still very effective. A
substance, usually saccharin, is used to tell if any outside air is seeping
threw the seal of the mask. A hood is
placed over the subject to keep the saccharin released in the air in the
vicinity of the mask so it may be detected.
First the person administering the test releases the saccharin into the
hood while the person is not wearing a mask.
This is to see how much saccharin is needed to be administered before it
can be detected. Than the person drinks
water to clear the taste from there mouth.
The mask is than fitted onto the persons face. Saccharin is than added to the closed hood
once again. The person is asked to move
their head side to side, and up and down.
The person is asked to read “The Rainbow Passage”. The subject is asked to bend over or jog in
place as well. If the person at any
point detects the scent/taste during the test than you know the seal is not
good. The mask is than either refitted
or swapped for another type of respirator.
The process is similar for the other method as well.
The subject is still asked to read the
rainbow passage, move side-to-side, tilt up and down etc. A hose is fitted to the respirator and than
connected to the machine. While the person is performing the tasks previously
listed, the machine measures the amount of particulate entering the air the
person is breathing within the mask. The
same process is repeated as it is in the smell/taste test. The mask is either refitted or swapped out if
the subject fails the test. One big
reason a person may fail the test is if they have facial hair that prevents the
mask from sealing to there face. Many
times the employee may be asked to shave their face to ensure a better fit. Naturally, this is usually more of a problem
with men.
Monday, March 14, 2016
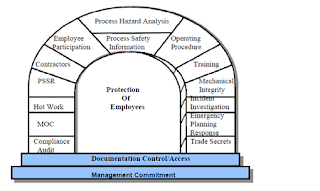
Process Safety Management (PSM)
Process
safety management or (PSM) is an analytical tool centered on preventing
releases of any substance defined as a highly hazardous chemical. PSM refers to a set of approaches that are used to
manage hazards associated with the industrial processes and it is intended to
reduce the frequency and severity of incidents resulting from releases of
chemicals and other energy sources (US OSHA 1993). These standards are composed
of organizational and operational procedures, design guidance, audit programs,
and convey of other methods. Here I have included the 14 step process and
an explanation from OSHA’s website.
1) Develop and maintain written safety information identifying workplace
chemical and process hazards, equipment used in the processes, and technology
used in the processes.
(2) Perform a workplace hazard assessment,
including, as appropriate, identification of potential sources of accidental
releases, identification of any previous release within the facility that had a
potential for catastrophic consequences in the workplace, estimation of
workplace effects of a range of releases, and estimation of the health and
safety effects of such a range on employees.
(3) Consult with employees and their
representatives on the development and conduct of hazard assessments and the
development of chemical accident prevention plans and provide access to these
and other records required under the standard.
(4) Establish a system to respond to
the workplace hazard assessment findings, which shall address prevention, mitigation,
and emergency responses.
(5) Review periodically the workplace hazard assessment
and response system.
(6) Develop and implement written
operating procedures for the chemical processes, including procedures for each
operating phase, operating limitations, and safety and health considerations.
(7) Provide written safety and
operating information for employees and employee training in operating
procedures, by emphasizing hazards and safe practices that must be developed
and made available.
(8) Ensure contractors and contract
employees are provided with appropriate information and training;
(9) Train and educate employees and
contractors in emergency response procedures in a manner as comprehensive and
effective as that required by the regulation promulgated pursuant to section
126(d) of the Superfund Amendments and Reauthorization Act.
(10) Establish a quality assurance
program to ensure that initial process-related equipment, maintenance
materials, and spare parts are fabricated and installed consistent with design
specifications; Process Safety Management 4.
(11) Establish maintenance systems for
critical process-related equipment, including written procedures, employee
training, appropriate inspections, and testing of such equipment to ensure ongoing
mechanical integrity.
(12) Conduct pre-startup safety reviews
of all newly installed or modified equipment.
(13) Establish and implement written
procedures managing change to process chemicals, technology, equipment and
facilities.
(14) Investigate every incident that results
in or could have resulted in a major accident in the workplace, with any
findings to be reviewed by operating personnel and modifications made, if
appropriate.
Process Safety has developed over
the years. Unfortunately, this is often
due to severe incidents. But it is
through these incidents that we learn.
OSHA and the EPA have a huge influence on the PSM. OSHA, covering the safety of the employees. And the EPA covering the environmental risks
that industry causes. However the two
can be heavily tied, for example the Bhopal, India release was an environmental
pollutant that cause a lot of harm to people (not necessarily employees in this
case). This is just one example of how the
two are heavily intertwined.
Thursday, February 18, 2016
t-butyllithium in heptane
Shipping hazardous materials
of any sort poses a massive risk to people.
The people most at risk is the person packaging the material, the
person transporting it, and the person opening the package. However certain materials can put large
amounts of people in danger. In this
scenario, we are going to talk about transporting a very hazardous
chemical. The chemical is known as t-butyl-lithium in heptane (heptane is the solvent). This is classified as a pyrophoric liquid. Pyrophoric
chemicals are
liquids and solids that have the potential to spontaneously ignite in air at
temperatures of 130 degrees Fahrenheit/ 54 degrees Celsius or below. It also
has corrosive, water reactive, and peroxide forming properties. TBL (t-butyl-lithium) will catch fire if exposed
to oxygen/air. If exposed to water, TBL reacts
very violently and gives off flammable gases and corrosive dust. TBL is so reactive with water; even the
moisture from your body or skin will cause it to react, if the oxygen in the
air has not already caught it on fire.
Inhaling the vapors from TBL is very dangerous.
Inhalation of vapors may cause dizziness,
nausea, anesthesia, numbness, motor weakness in fingers and toes,
incoordination, and headaches. If
ingested, TBL may produce a lung aspiration.
As you can see, this chemical is very dangerous in many ways. When shipping this chemical, it is to be kept
free of contact from water, air, and oxidizing materials. When being handled, personnel should wear a
full-face mask and gloves at the very least.
The environment in which TBL is being handled should be a closed system
under argon and nitrogen gases. TBL
should be kept away from any sparks and flames. Storage containers should be protected, and
physically inspected for leaks and physical damage. Shipping TBL should be very carefully carried
out. The package containing TBL should show that it
is an organometallic substance, that it is a liquid, that it is pyrophoric, and
that it is water reactive. The package
should also be labeled “T-BUTYLLITHIUM, HYDROCARBON SOLUTION, 4.2(4.3), UN 3394, PG I”. Shipments also require a
“Dangerous When Wet” and “Spontaneously Combustible” label(s). Transport of TBL by post, parcel, and air,
are prohibited in the United States. It
is however aloud to be transported by roadway and railway in class
4.2(4.3)(DOT). All shipments on roadways
need to be carried out in a DOT(Department of Transportation) approved vehicle. It is also aloud to be transported by sea in
class 4.2(4.3)(IMDG). When shipping TBL
the container is not to be filled more than 90 percent of its potential
capacity. The extra space in containers
is filled with an inert gas like nitrogen.
Glass containers are fitted with a septum so the chemical can be
retrieved with a syringe. Other
containers are slightly pressurized and fitted with one-way valves to prevent
air contamination. Containers should be
air and watertight. Containers should
also have an extremely robust outer shell to ensure the TBL does not
escape. More than one layer of
containment is highly recommended due to the extreme danger TBL poses. Vehicles carrying the chemical should be
clearly marked as hazardous material transporters.
Subscribe to:
Comments (Atom)